Equation of State of a Perfect Gas
Equation of State of a Perfect Gas: Overview
This Topic covers sub-topics such as Boyle's Law, Charles's Law, Avogadro's Law, Gay-Lussac's Law, Gaseous State, Ideal Gas Assumptions, Dalton's Partial Pressure Mixture Law and, Equation for State for Ideal Gas
Important Questions on Equation of State of a Perfect Gas
of a gas at a pressure of is compressed to . Taking the temperature to remain constant, the increase in pressure, is
Under what conditions will a pure sample of an ideal gas not only exhibit a pressure of but also a concentration of ?
Select one correct statement. In the gas equation, PV = nRT
A container of an ideal gas that is isolated from its surroundings is divided into two parts. One part has double the volume of the other. The pressure in each part is and the temperature is the same. The partition is removed. What is the pressure in the container now?
Significance of and in Van der Waals equation:
Equation for an ideal gas is:
By what percentage should the pressure of a given mass of gas be increased to decrease its volume by at a constant temperature?
The number of molecules in of a gas at pressure and temperature will be,
Two vessels contain two different ideal gases and at same temperature. If the ratio of molecular weights of and is then ratio of densities of and , if ratio of pressure is is
moles of an ideal diatomic gas with initial temperature is compressed from to . The thermodynamic process is such that , where is a constant. Then, the value of is close to (the gas constant, ).
The internal energy of a gram-molecule of an ideal gas depends on _______.
The volume of a certain mass of gas at constant pressure is doubled to its value at . The temperature of the gas will be,
The equation of state for of at pressure and temperature at volume will be :
During an experiment an ideal gas is found to obey an additional law constant. The gas is initially at temperature and volume when it expands to volume the resulting temperature is:-
For one gram molecular weight of a gas, the value of in the equation is nearly,
A gas at the temperature is contained in a closed vessel. If the gas is heated through then the percentage increase in its pressure will be
One mole of monoatomic gas is brought from state to state . The initial temperature at is . The temperature at will
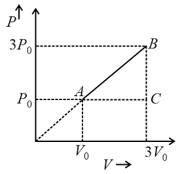
Three containers of the same volume contain three different gases. The masses of the molecules are and and the number of molecules in their respective containers are and . The gas pressure in the containers are and respectively. All the gases are now mixed and put in one of the containers. The pressure of mixture will be :-
For next two question please follow the same
The figure shows a V - T graph of process carried on one mole of mono atomic gas. The slope of the graph in the process CA varies as where is a constant and p is the pressure of the gas.
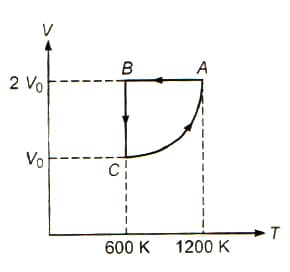
The relation between temperature and pressure variation for the process CA is
The equation shows the relationship between a variety of gas properties. What is this equation called?
